During rotary kiln baking, operating manuals always emphasize “the heating rate must not be too fast.” Why? Because there exists an easily overlooked physical phenomenon inside castables—a steam pressure buildup effect similar to a “pressure cooker.”Below is a detailed explanation.
Hydration Reactions of Calcium Aluminate Cement
Calcium aluminate cement is a critical component of castable refractories. The reason is simple: when calcium aluminate cement reacts with water, it produces hydration products. These hydration products give the castable its strength. Here’s how the mechanism works:
Calcium aluminate cement contains three main mineral phases: CA (monocalcium aluminate), CA₂ (dicalcium aluminate), and C₁₂A₇ (dodecacalcium heptaaluminate). At room temperature, these phases react with water. The reaction generates various hydration products. These products give the castable its initial strength. That’s why freshly installed castables require a curing period. See the diagram below for the chemical reactions.
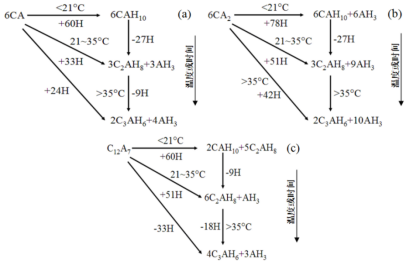
Decomposition of Hydration Products and Strength Changes
The hydration products of calcium aluminate cement decompose at different temperatures. The low-temperature stable hydration phase CAH₁₀ decomposes at around 120°C. C₂AH₈ decomposes at approximately 170–195°C (you can only observe direct decomposition if you heat rapidly after low-temperature curing). After baking at 110°C, the main cement hydration phases are C₃AH₆ and AH₃. Between 250–300°C, these two phases decompose into C₁₂A₇ and AlO(OH) respectively.
The early strength of castables comes mainly from the hydration products of calcium aluminate cement. Between 120°C and 300°C, these hydration products gradually decompose. This causes the structure of the hydration products to collapse. As a result, the castable’s strength drops significantly. That’s why castables have lower strength in this temperature range.
Steam Pressure Cracking Mechanism During Rotary Kiln Baking
In addition, the hydration reaction of calcium aluminate cement is a dissolution-precipitation reaction. The calcium aluminate cement first dissolves in water. Then it recrystallizes to form hydration products wherever space is available inside the castable. This causes the hydration products to block the internal capillary pores of the castable. As a result, many enclosed spaces form inside.
During the rotary kiln baking process, free water and bound water inside the castable turn into steam as temperature rises. The higher the temperature, the greater the saturated vapor pressure of the steam generated.
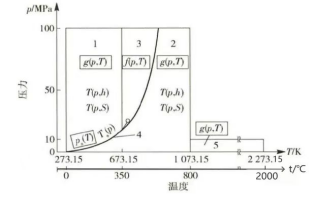
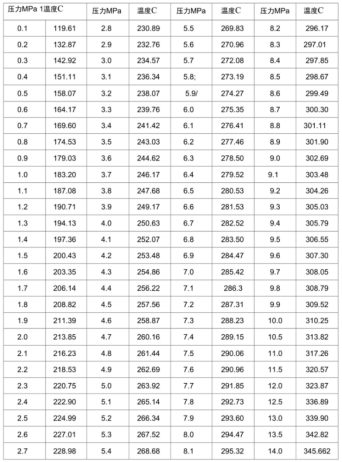
As shown in the table above,at 120°C, the saturated vapor pressure of steam is 0.1 MPa. At 200°C, it reaches 1.5 MPa. When temperature continues to rise to 264°C, the saturated vapor pressure becomes 5 MPa. At 300°C, it climbs to 8.7 MPa. At 345.7°C, the saturated vapor pressure reaches 14 MPa. Although the flexural strength of castables can reach 6 MPa or higher after baking at 110°C, water turns into steam in enclosed spaces inside the castable. When water converts to steam, its volume changes dramatically. According to pv=nRt, at 200°C, steam occupies 2,157 times the volume of the same mass of water. When water inside the castable turns to steam, the volume expands rapidly. At the same time, hydration products begin to decompose. The structure of these hydration products collapses. The steam pressure generated internally can crack the castable from within at just 1-2 MPa. Therefore, castables typically require extended holding periods at 110°C and 240-250°C.







